Abstract
Background
Systemic light-chain amyloidosis (AL) is a life-threatening disease caused by the misfolding and deposition of clonal free light-chains (FLC). The current standard of therapy is targeting the malignant plasma cells with chemotherapy to reduce the circulating FLC burden.
Daratumumab is a human monoclonal IgG kappa antibody targeting CD38 which is mainly expressed on plasma cells. It has been approved as a monotherapy in the European Union in May 2016 for treatment in patients with multiple myeloma (MM) exposed to proteasome inhibitors (PI) and immunomodulatory drugs (IMID). Sher et al. (Blood 2016) first published a case report with an FLC response in a patient with cardiac AL and a hematologic complete remission (CR) in a patient with renal AL. Kaufman et al. (Blood 2017) reported a hematologic response (>PR) in 19 of 25 pretreated patients with systemic AL.
Patients and Methods
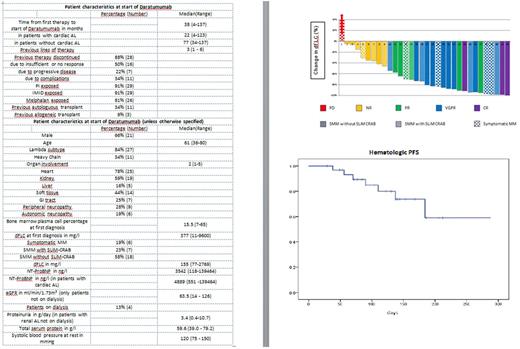
This is a retrospective analysis of 32 consecutive patients treated with daratumumab monotherapy starting from October 2016 for symptomatic AL at our institution. Only patients with an underlying MM (smoldering or CRAB+) were initiated on daratumumab. Hematologic remission and NT-ProBNP response were assessed according to Palladini et al., JCO 2012, renal response according to Palladini et al., Blood 2014. Daratumumab 16 mg/kg of total body weight was applied intravenously after premedication with dexamethasone (commonly 20 mg), intravenous paracetamol and antihistamine. Infusions were initiated weekly in 17 patients and every other week in 15 patients due to reduced performance status. For evaluation of hematologic progression-free survival (PFS) the addition of a second therapeutic agent was also considered as an event. Hematologic PFS was calculated using SPSS v.24.0 statistical software (IBM, Armonk, New York, USA)
Patient cohort: For patient characteristics please refer to the tables .
Results
Hematologic response: Twenty-three of 32 patients achieved a hematologic remission after a median duration of 49 days (range: 5-287). Patients received a median of 8 (range: 2-17) infusions of daratumumab. CR was achieved in 3 patients, a very good partial remission (VGPR) in 14 and a partial remission (PR) in 6 patients (see figure).
The 3 month overall response rate among 27 evaluable patients was 63% with CR in 3 patients, VGPR in 10 patients and PR in 4 patients.
Progression and survival: After a median follow-up time for patients alive of 134 days from the first daratumumab infusion median PFS is not reached (figure). Currently, 21 patients are still on and four patients are off therapy without hematologic progress. One patient died from a cardiac event after discontinuing daratumumab for no response. Another patient with a VGPR died after infectious complications.
Organ response at 3 months: For 14 of 25 patients with cardiac AL NT-ProBNP analysis was performed. Two patients had an NT-ProBNP response and six patients had an NT-ProBNP increase. Renal response assessment was performed in 9 of 15 patients with renal involvement not on dialysis and showed a response in 3 patients and a progression in 3 patients.
Complications: One patient with cardiac AL and terminal renal failure on peritoneal dialysis died after developing peritonitis after 90 days of first daratumumab infusion. Another four patients with cardiac AL were admitted to hospital for lower respiratory tract infections (3) or cardiac decompensation (1). Mild upper respiratory tract infections were reported in six patients. One patient newly developed atrial fibrillation. Two patients with symptomatic MM experienced dyspnoea during the first infusion. One patient with symptomatic MM required a blood transfusion.
Summary
Daratumumab monotherapy can induce fast hematologic responses in heavily pre-treated AL patients who cannot tolerate or are refractory to proteasome inhibitors or immunomodulatory drugs. NT-ProBNP and renal responses can be achieved, although NT-ProBNP progression under therapy was common in our cohort. As expected, patients with advanced cardiac AL are at a higher risk of severe complications.
Schönland: Sanofi: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; prothena: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau. Hegenbart: Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Prothena: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal